An Apiject prefilled single-dose injector, manufactured using Blow-Fill-Seal technology. (Image courtesy PRNewsfoto/Apiject Systems, Corp.)
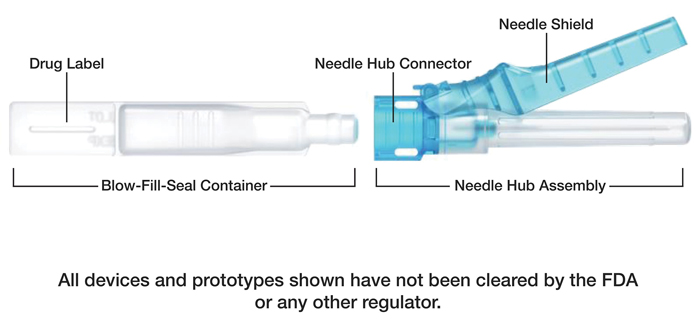
The company’s drug delivery development platform integrates its Blow-Fill-Seal liquid packaging technology and precision injection molding of pen-style needle hubs.
STAMFORD, Conn.—Apiject Systems, Corp. describes itself as a public-benefit medical technology company, pioneering a new category of prefilled injection devices that are reported to offer “economic, supply chain, and sustainability advantages over traditional offerings.”
“Our mission is to make the safety and performance benefits of prefilled injections affordable and available for most, if not all, injections around the world,” the company stated in a release.
Apiject Systems recently submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for regulatory approval of the drug Glycopyrrolate, which it called “the world’s first injectable medicine to use Apiject’s single dose, single use prefilled plastic syringe.”
At the center of Apiject’s NDA submission is a drug delivery development platform that integrates two proven medical manufacturing technologies—Blow-Fill-Seal (BFS) liquid packaging technology and precision injection molding of pen-style needle hubs, according to a release from Apiject Systems.
Apiject’s Blow-Fill-Seal (BFS) is a sterile liquid packaging technology that has been recognized by the FDA as an advanced aseptic process. It is a continuous manufacturing process that operates at high speed and relies on a single, domestically available raw material. The combination of BFS and precision injection molding enables creation of a new category of prefilled drug delivery devices that are said to be “more scalable and affordable than traditional glass vials and prefilled syringes.”
“Today’s submission is an exciting and significant step forward for Apiject,” said Apiject Systems Co-founder, Executive Chairman, and CEO Jay Walker, in a statement. “We have spent the last five years preparing BFS to play a central role in the future of drug delivery, because it meets the needs of the new challenges of today’s healthcare realities. Our work has involved extensive drug container and device design and development, building enhancements to the manufacturing process, inventing new equipment, as well as extensive end-to-end testing. The global demand for medical injections continues to grow at a double-digit pace. We need new domestic capacity, more flexibility, lower costs, and we need new thinking.
“Simplified manufacturing, compact supply chains, flexible production that can scale quickly, reduced foreign dependencies, lower carbon output, and more affordable prefilled solutions are what the U.S. and the world needs right now—for commercial use, public health campaigns, and emergency response,” Walker added. “And that’s exactly the kind of innovation we are focused on at Apiject.”
Blow-Fill-Seal is highly flexible in container design and sizes to accommodate a wide range of medical uses, including eye drops, ear drops, nasal drops, wound care, and sterile water. Now, it will be used for injections, and soon, inhalants and infusions.
In 2004, the FDA formally recognized BFS as an advanced aseptic process. In its guidance document, “Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice,” the FDA defined BFS as an “automated process by which containers are formed, filled, and sealed in a continuous operation.”
With BFS at its core, the Apiject system is reported to reduce costs, complexity, and supply chain risks. It is said to offer a significant improvement over the multi-step, multi-factory process of making and then filling glass drug containers.
“Manufacturing the Apiject device creates less than half the carbon output of a glass vial and syringe, and even uses less plastic than the traditional syringe itself,” the release stated.
Apiject’s underlying technology was developed, in part, through an Other Transaction Agreement and Contract for approximately $181 million from the U.S. Department of Health and Human Services (HHS), Administration for Strategic Preparedness and Response (ASPR) during President Trump’s first term in office, the company said.
“The project highlights America’s renewed commitment to accelerate the building of new U.S.-based, high-speed, population-scale capacity for pharmaceutical manufacturing that reduces the country’s dependence on foreign suppliers, most notably China and India,” according to the release.
Under the agreement with HHS-ASPR, and with the contract management support of the U.S. Department of Defense, Apiject delivered an emergency domestic fill-finish capacity, on-time and on-budget, that served as a backup in the event of critical supply disruptions for traditional injection materials. Throughout the development of its technology and device platform, Apiject has been supported by an equal amount of private sector investment, making this a true public-private partnership.
As a medical technology company, Apiject works with pharmaceutical organizations to design and help manufacture scalable and affordable prefilled drug delivery systems that enable injectable medicines and vaccines to reach more patients across the world. Apiject’s simple, lightweight, and sterile BFS Container and Needle Hub with Connector are said to be easily push-assembled by a healthcare professional just prior to patient injection.
Apiject stated that it recently announced a strategic collaboration with Amneal Pharmaceuticals, an integrated U.S.-based biopharmaceutical company, to expand domestic production of Apiject’s BFS-based injectable platform at Amneal’s Brookhaven, New York facility.
“Almost five years of intensive R&D has prepared BFS—drug containers, devices, process equipment—to play a critical role in Apiject’s innovative platform across all major routes of injectable drug administration,” said Apiject Chief Operating Officer and NDA Project Leader Molly Weaver, Ph.D., in a statement. “Our innovation team, led by Apiject co-founder Marc Koska, the inventor of the K-1 auto-disabled syringe credited with saving millions of lives across the globe, together with our device and process teams focused on product development, quality, and regulatory compliance, have worked hand-in-hand to create this technology and its application for public health.”
Glycopyrrolate is indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer by reducing the amount of acid produced. Apiject’s device is designed to be drug agnostic, and this NDA is the first of future combination product filings leveraging the Apiject system currently in development.
Other projects in development using Apiject technology are reported to include a nasal delivery device filled with Naloxone to treat opioid overdoses of fentanyl.
